
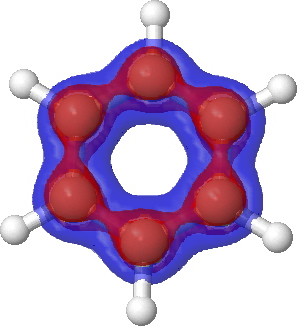
A bond formed in this way is called a pi bond.įor clarity, the sigma bonds are shown using lines - each line representing one pair of shared electrons. In this one the electrons aren't held on the line between the two nuclei, but above and below the plane of the molecule. This sideways overlap also creates a molecular orbital, but of a different kind. Notice that the p orbitals are so close that they are overlapping sideways. In the diagram, the black dots represent the nuclei of the atoms. The p orbitals on each carbon aren't pointing towards each other, and so we'll leave those for a moment. Molecular orbitals made by end-to-end overlap of atomic orbitals are called sigma bonds.
The various atomic orbitals which are pointing towards each other now merge to give molecular orbitals, each containing a bonding pair of electrons. The two carbon atoms and four hydrogen atoms would look like this before they joined together: The remaining p orbital is at right angles to them.

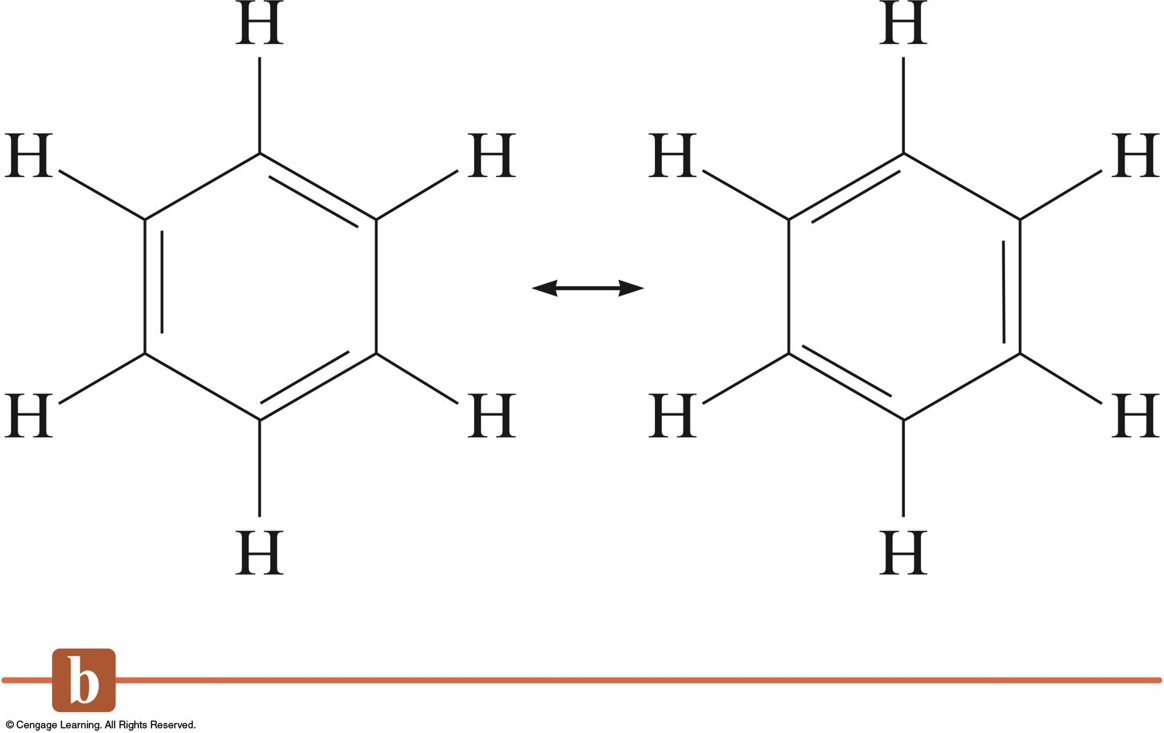
The three sp 2 hybrid orbitals arrange themselves as far apart as possible - which is at 120° to each other in a plane. sp 2 orbitals look rather like sp 3 orbitals that we discussed in the bonding in methane in the page on single bonds, except that they are shorter and fatter. The new orbitals formed are called sp 2 hybrids, because they are made by an s orbital and two p orbitals reorganising themselves. It's because it uses the orbitals with the lowest energy first. Note: You might wonder why it chooses to hybridise these three orbitals rather than just use the three p orbitals which already have the same energy. They use the 2s electron and two of the 2p electrons, but leave the other 2p electron unchanged. When the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise three of the orbitals rather than all four. In the case of ethene, there is a difference from methane because each carbon is only joining to three other atoms rather than four. The carbon atom is now in an excited state. This is exactly the same as happens whenever carbon forms bonds - whatever else it ends up joined to. The carbon atom doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s 2 pair into the empty 2p z orbital. Unless you have some understanding of the true nature of the double bond, you can't really understand the way that ethene behaves.Įthene is built from hydrogen atoms (1s 1) and carbon atoms (1s 22s 22p x 12p y 1). It is important to explore the bonding in ethene in more detail because it has a direct impact on its chemistry. Each line represents one pair of shared electrons.Įthene has a double bond between the two carbon atoms.Ī more sophisticated view of the bonding in ethene The double bond is shown conventionally by two lines joining the atoms. Two oxygen atoms can both achieve stable structures by sharing two pairs of electrons as in the diagram. Some simple molecules containing double bonds If you have come straight to this page via a search engine follow this link before you go on.Ī double covalent bond is where two pairs of electrons are shared between the atoms rather than just one pair. Warning! This page assumes that you have already read the page on single covalent bonds. It starts with a simple picture of double covalent bonding, and then takes a more sophisticated view of the bonding in ethene. This page explains how double covalent bonds arise.


 0 kommentar(er)
0 kommentar(er)
